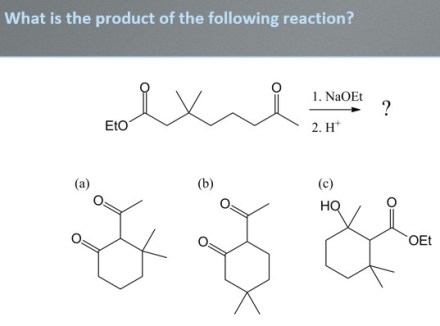
The other day, while working through Chapter 21, I left class with a great feeling as a result of having given the following clicker question:
My students were able to rule out choice (a) on their own, given that there’s no reasonable mechanism to arrive at that product with the methyl groups located there. However, they had trouble choosing between (b) and (c). I wasn’t surprised at their struggle because those compounds are the products of two similar mechanisms: (b) is the product of an intramolecular Claisen-type reaction and (c) is the product of an intramolecular aldol addition. Furthermore, each reaction has produced a relatively stable six-membered ring. So if my students had difficulty with this question, why did I leave class with a great feeling? It was because of what happened next.
When my students asked why (b) was the major product instead of (c), I explained it to them in the following way. I drew out the complete mechanism for the reactions leading to (b) and (c). I reminded my students that the aldol addition reaction leading to (c) is reversible and, more to the point, somewhat unfavorable because the enolate adds to a ketone. I also reminded them that the Claisen-type reaction leading to (b) is reversible and unfavorable up to the formation of the initial 1,3-dicarbonyl, but the subsequent deprotonation is irreversible. In essence, we can view the aldol product and the initial 1,3-dicarbonyl product as being in equilibrium and the irreversible deprotonation removes the 1,3-dicarbonyl from the equilibrium:
LeChâtelier’s principle dictates that the equilibrium continually shifts to produce more of the 1,3-dicarbonyl and therefore pulls the entire equilibrium toward the Claisen-type product, (b).
At times throughout my explanation, I wondered if my students were fully grasping things. After all, 15 years ago when I was teaching using a traditional functional group book, my students struggled mightily with even the simplest of mechanisms. In the problem at hand, I was taking for granted that my students were comfortable with the mechanisms, and I was engaging my students on a higher plane. Each time I looked at my students, though, I was greeted with the affirmative head nods. They were “getting it.” I realized that my teaching had come a long way since 15 years ago and that what I had believed all along was reaffirmed: teaching under a mechanistic organization does not require high-level students, it produces them.
-Joel Karty